Acanthus Pharma Lipids
Acanthus Pharma chemistry group now offers developmental
services for customized lipids used in lipid nanoparticles for drug
delivery and vaccines
We tailor our chemistry process to meet your requirements.
- Energy Storage: Triglycerides and other lipids effectively store energy in adipose tissue.
- Phospholipids and cholesterol are essential components of cell membranes that give them shape and fluidity.
- Signaling Molecules: Steroid hormones and prostaglandins are examples of lipids that function as signaling molecules, controlling different physiological processes.
- Insulation and Protection: Lipids act as insulation to keep the body at a constant temperature and safeguard important organs.
Benefits of lipid-based formulations:
- Enhanced Solubility: Lipid-based formulations, such as liposomal doxorubicin, improve the chemotherapeutic drug’s solubility, which lessens adverse effects and increases its efficacy. [1]
- Protection and Stability: The lipid nanoparticles included in mRNA vaccines, including the COVID-19 and Pfizer-BioNTech vaccines, shield the mRNA from deterioration and guarantee its stability up until vaccination. [2]
- Controlled Release: Medicines like ibuprofen can be delivered using solid lipid nanoparticles (SLNs), which offer controlled release and prolong the time that pain alleviation lasts. [3]
- Targeted Delivery: Drugs like Doxil, which targets malignant tissues more efficiently than traditional formulations, can be precisely delivered to cancer cells using lipid-coated nanoparticles. [4]
- Versatility: The therapeutic efficacy of hydrophobic medicines, such as paclitaxel (Taxol), can be increased by using lipid-based micelles to deliver the drug in a more soluble and bioavailable form. [5]
- Customized Formulations: Drug delivery, such as insulin for diabetes control, can be optimized by customizing nanostructured lipid carriers (NLCs) for medications and therapeutic needs. [6]
Working Together. Smarter and Faster.
Lipids
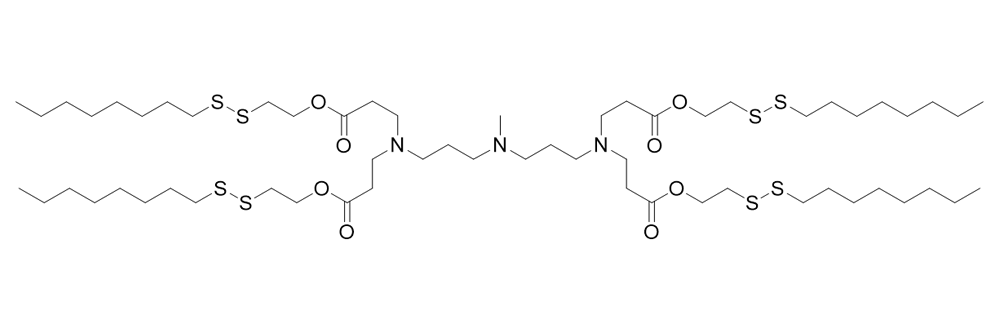
306-O12B
Catalog No: APS-LP-CAT-11
Name: 306-O12B
CAS No: 2566523-06-4
Type: Cationic/Ionizable
Grade: R & D or GMP
TT3
Catalog No: APS-LP-CAT-04
Name: TT3
CAS No: 1821214-50-9
Type: Cationic/Ionizable
Grade: R & D or GMP
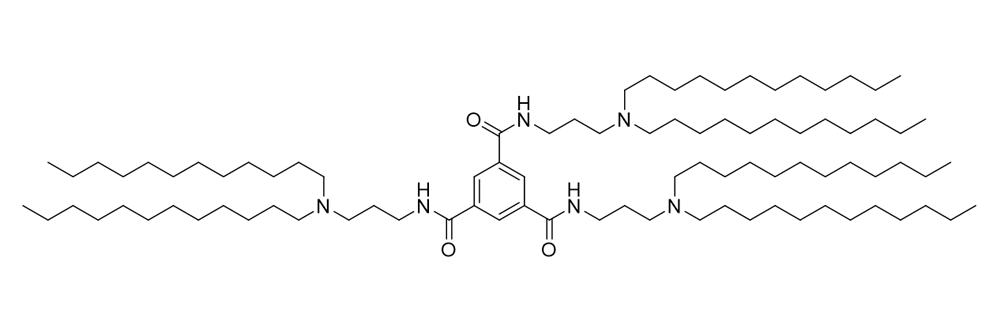
LP-01
Catalog No: APS-LP-CAT-16
Name: LP-01
CAS No: 1799316-64-5
Type: Cationic & Ionizable
Grade: R & D
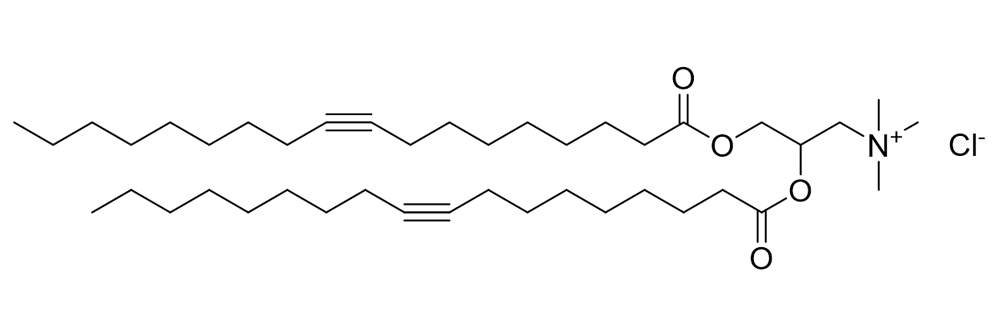
DOTAP-Cl
Catalog No: APS-LP-CAT-06
Name: DOTAP-Cl
CAS No: 132172-61-3
Type: Cationic/Ionizable
Grade: R & D or GMP
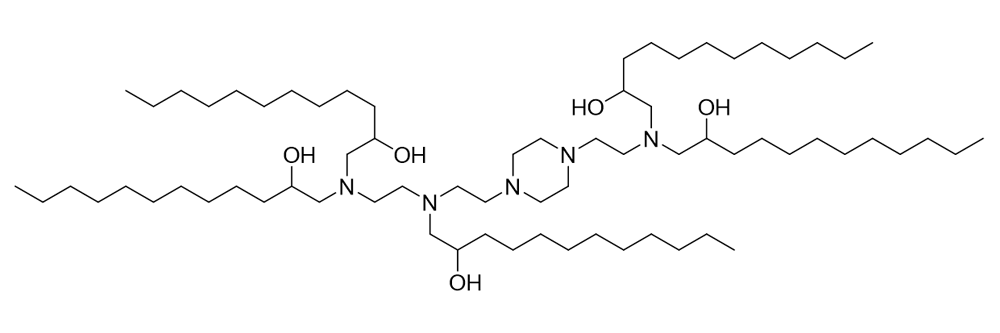
C12-200
Catalog No: APS-LP-CAT-07
Name: C12-200
CAS No: 1220890-25-4
Type: Cationic/Ionizable
Grade: R & D
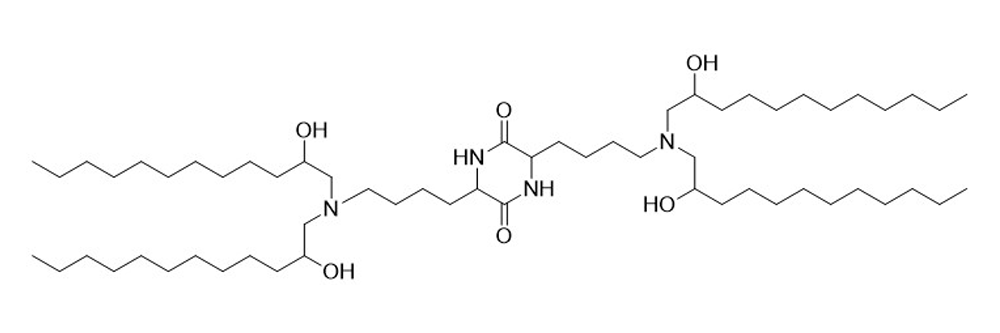
cKK-E12
Catalog No: APS-LP-CAT-08
Name: cKK-E12
CAS No: 1432494-65-9
Type: Cationic/Ionizable
Grade: R & D or GMP
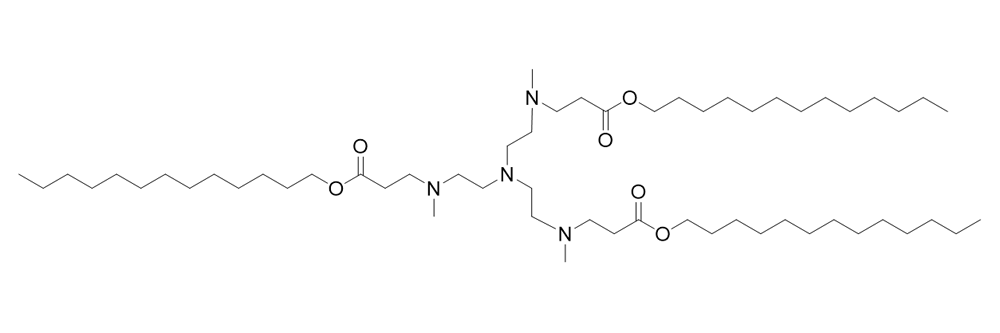
304O13
Catalog No: APS-LP-CAT-10
Name: 304O13
CAS No: 1566559-80-5
Type: Cationic/Ionizable
Grade: R & D or GMP
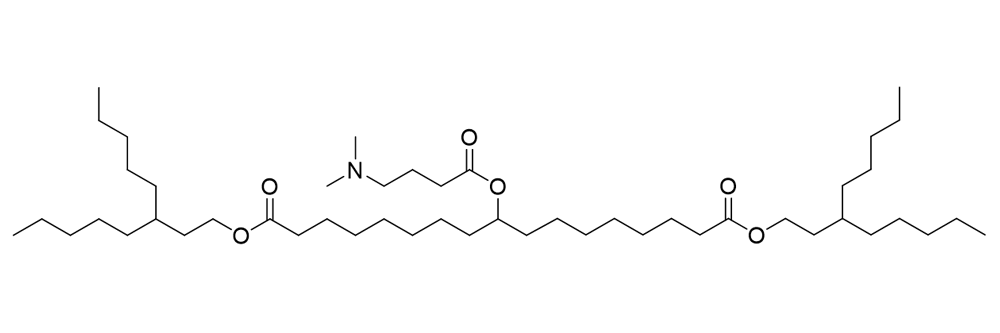
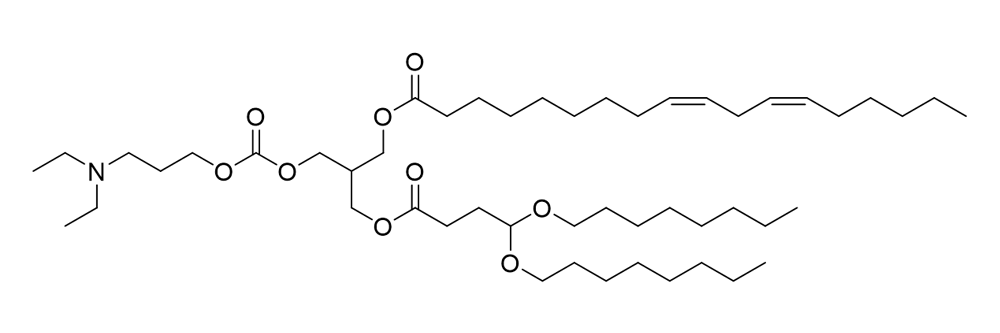
O-10461
Catalog No: APS-LP-CAT-12
Name: O-10461
CAS No: 1443522-24-4
Type: Cationic/Ionizable
Grade: R & D or GMP
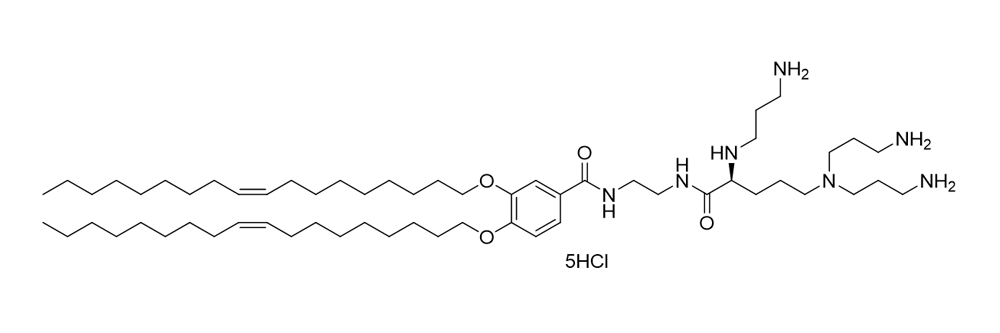
MVL5
Catalog No: APS-LP-CAT-14
Name: MVL5
CAS no: 464926-03-2
Type: Cationic/Ionizable
Grade: R & D
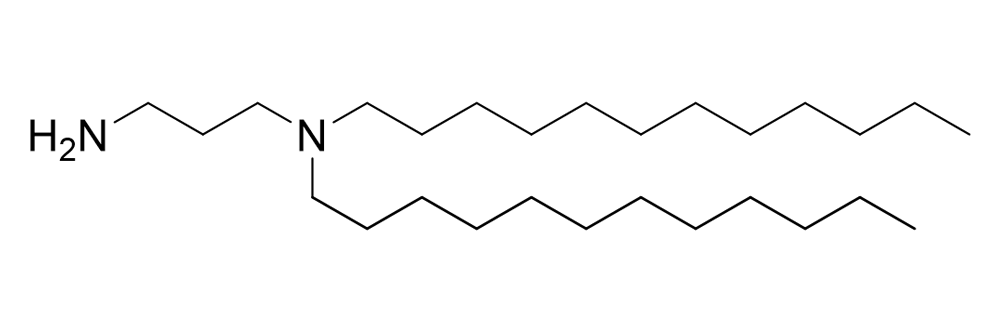
DDPDA
Catalog No: APS-LP-AIN-02
Name: DDPDA
CAS No: 2889340-25-2
Type: Advanced Lipid Intermediates
Grade: R & D or GMP
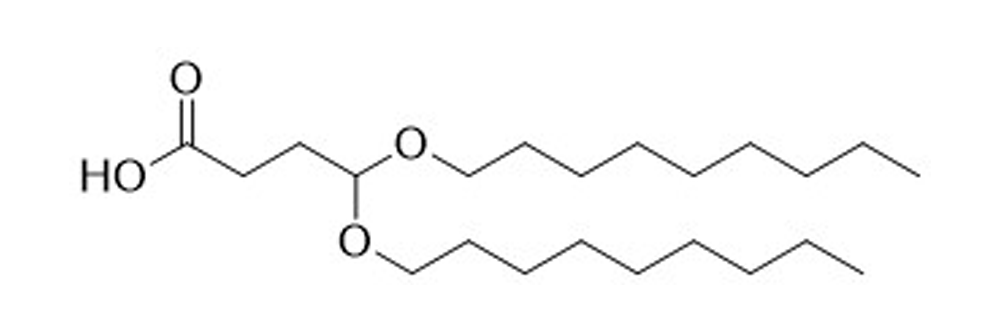
BNB-A
Catalog No: APS-LP-INT-02
Name: BNB-A
CAS No: 2305258-93-7
Type: Advanced Lipid Intermediates
Grade: R & D or GMP
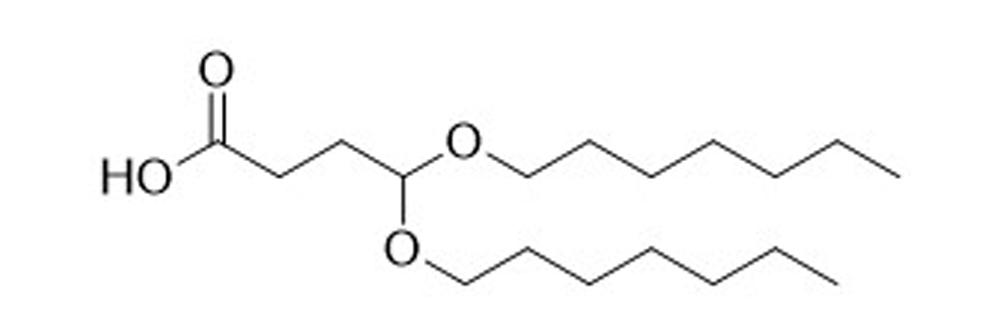
BHeptB-A
Catalog No: APS-LP-INT-03
Name: BHeptB-A
CAS No: 2305258-90-4
Type: Advanced Lipid Intermediates
Grade: R & D or GMP
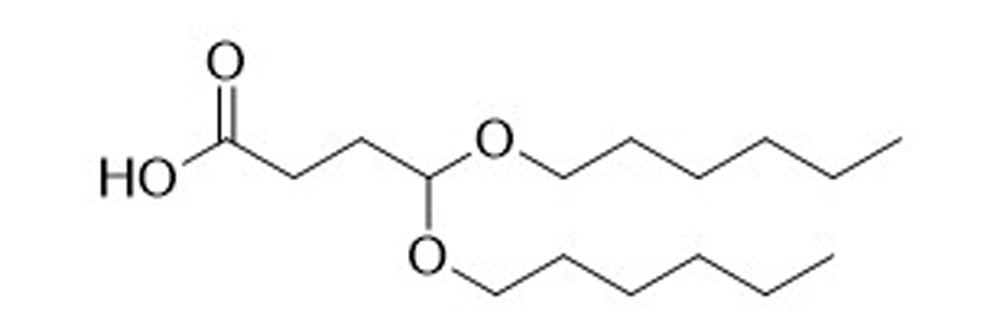
BHexB-A
Catalog No: APS-LP-INT-04
Name: BHexB-A
CAS No: 2305258-87-9
Type: Advanced Lipid Intermediates
Grade: R & D or GMP
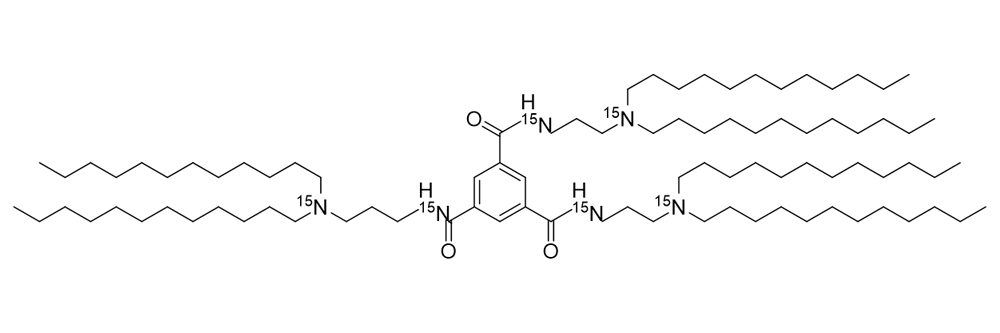
TT3 15N6
Catalog No: APS-LP-CAT-05
Name: TT3 15N6
CAS no: 1821214-50-9 (CAS for non-labelled material)
Type: Stable Isotope Labelled
Grade: R & D or GMP
Working Together. Smarter and Faster.
[1] Barenholz, Y. (2012). Doxil®—the first FDA-approved nano-drug: Lessons learned. Nature Reviews Drug Discovery, 9(12), 971-975. DOI: https://doi.org/10.1016/j.jconrel.2012.03.020
[2] Linde Schoenmaker, Dominik Witzigmann, Jayesh A. Kulkarni, Rein Verbeke, Gideon Kersten, Wim Jiskoot, Daan J.A. Crommelin. (2021) mRNA-lipid nanoparticle COVID-19 vaccines: Structure and stability, International Journal of Pharmaceutics, 601, 120586. DOI: https://doi.org/10.1016/j.ijpharm.2021.120586
[3] Müller, R. H., Mäder, K., & Gohla, S. (2000). Solid lipid nanoparticles (SLN) for controlled drug delivery—a review of the state of the art. European Journal of Pharmaceutics and Biopharmaceutics, 50(1), 161-177. DOI: https://doi.org/10.1016/s0939-6411(00)00087-4
[4] Gabizon, A., Shmeeda, H., & Barenholz, Y. (2003). Pharmacokinetics of pegylated liposomal Doxorubicin: Review of animal and human studies. Clinical Pharmacokinetics, 42(5), 419-436. DOI: https://doi.org/10.2165/00003088-200342050-00002
[5] Pandita D, Ahuja A, Lather V, Benjamin B, Dutta T, Velpandian T, Khar RK. Development of lipid-based nanoparticles for enhancing the oral bioavailability of paclitaxel. AAPS PharmSciTech. 2011 Jun;12(2):712-22. DOI: https://doi.org/10.1208%2Fs12249-011-9636-8
[6] Ilyas U, Asif M, Wang M, Altaf R, Zafar H, Faran Ashraf Baig MM, Paiva-Santos AC, Abbas M. Nanostructured Lipid Carrier-Based Delivery of Pioglitazone for Treatment of Type 2 Diabetes. Front Pharmacol. 2022 Jul 12;13:934156. DOI: https://doi.org/10.3389%2Ffphar.2022.934156
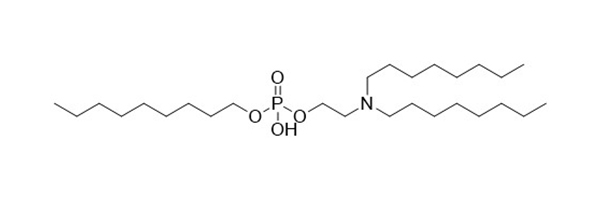
DOTAP-Cl
Sr no: 4
Name: DOTAP-Cl
Cas #: 132172-61-3
Type: Cationic & Ionizable
Grade: R & D/GMP

C12-200
Sr no: 5
Name: C12-200
Cas #: 1220890-25-4
Type: Cationic & Ionizable
Grade: R & D/GMP

CKK-E12
Sr no: 6
Name: CKK-E12
Cas #: 1432494-65-9
Type: Cationic & Ionizable
Grade: R & D/GMP